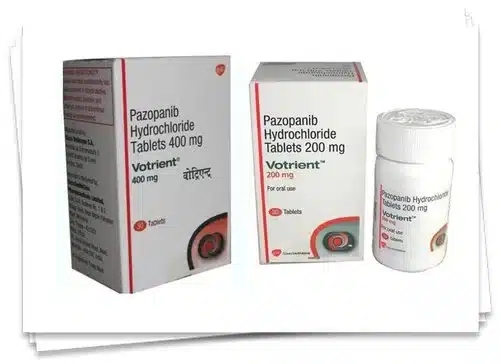
PAZOPANIB HYDROCHLORIDE (BDPAZO) Tablet
$189.00
Pazopanib Hydrochloride BDPAZO 200/400mg Tablet represents a significant advancement in the management of advanced RCC, STS, and other malignancies. By selectively targeting key signaling pathways implicated in tumor growth and angiogenesis, Pazopanib offers a tailored and effective therapeutic option to improve patient outcomes and enhance overall quality of life.
Description
Pazopanib Hydrochloride (BDPazo) Tablet is an oral medication used in the treatment of advanced renal cell carcinoma (RCC), advanced soft tissue sarcoma (STS), and certain other types of cancer. It belongs to the class of tyrosine kinase inhibitors (TKIs) and works by inhibiting the activity of multiple receptor tyrosine kinases (RTKs) involved in tumor angiogenesis and progression.
Key Features:
- Tyrosine Kinase Inhibitor: Pazopanib Hydrochloride inhibits the activity of various receptor tyrosine kinases (RTKs), including vascular endothelial growth factor receptors (VEGFRs), platelet-derived growth factor receptors (PDGFRs), and stem cell factor receptor (KIT). By blocking these signaling pathways, pazopanib disrupts tumor angiogenesis and inhibits tumor growth and metastasis.
- Treatment of Advanced RCC and STS: Pazopanib Hydrochloride (BDPazo) Tablet is indicated for the treatment of adult patients with advanced renal cell carcinoma (RCC) and advanced soft tissue sarcoma (STS) who have received prior chemotherapy. It offers a targeted treatment option for patients with these aggressive cancers.
- Oral Administration: Pazopanib Hydrochloride (BDPazo) Tablet is administered orally, typically once daily, with or without food. The convenient dosing regimen enhances patient compliance and adherence to treatment.
- Efficacy: Clinical trials have demonstrated the efficacy of pazopanib in prolonging progression-free survival and improving overall survival in patients with advanced RCC and STS. It has shown superiority over placebo and other conventional treatments in these patient populations.
- Safety Profile: Pazopanib generally has a manageable safety profile when used as directed. However, like all medications, it may be associated with certain side effects, including hypertension, diarrhea, fatigue, and hepatotoxicity. Patients should be closely monitored for adverse reactions during treatment.
- Patient Education: Patients prescribed Pazopanib Hydrochloride (BDPazo) Tablet should receive comprehensive education regarding proper medication administration, potential side effects, and the importance of adherence to treatment. This empowers patients to actively participate in their healthcare management and optimize therapeutic outcomes.
- Consultation with Healthcare Provider: Treatment with Pazopanib Hydrochloride (BDPazo) Tablet should be initiated and monitored by a qualified oncologist or healthcare provider experienced in the management of RCC and STS. Close monitoring and regular follow-up are essential to ensure optimal therapeutic response and patient safety.
- Access and Affordability: Efforts should be made to ensure equitable access to Pazopanib Hydrochloride (BDPazo) Tablet for eligible patients, including exploring financial assistance programs and insurance coverage options to mitigate any financial barriers to treatment. Access to targeted therapies like pazopanib is crucial for improving outcomes and quality of life for patients with advanced RCC and STS
Additional information
| Strength | 200mg, 400mg |
|---|
Only logged in customers who have purchased this product may leave a review.










Reviews
There are no reviews yet.