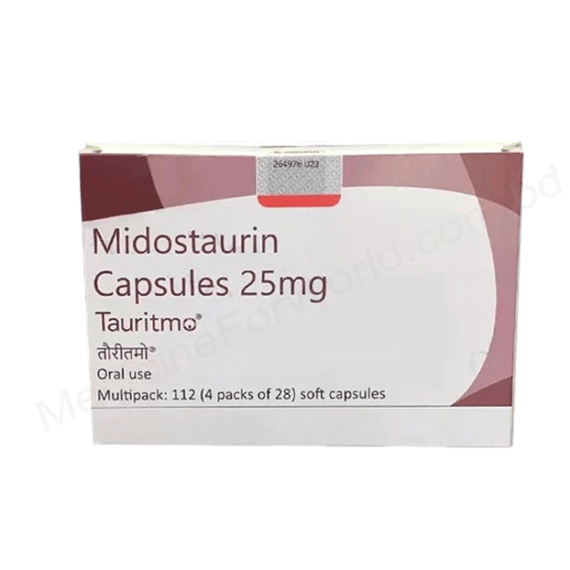
Midostaurin (TAURITMO CAP) 25MG Tablet
$354.00
TAURITMO Capsule offers a valuable treatment option for patients with FLT3-mutated AML and systemic mastocytosis, providing an effective and well-tolerated therapy to help manage these challenging hematologic malignancies.
Description
TAURITMO Capsule contains Midostaurin in a strength of 25mg, serving as a medication primarily prescribed for the treatment of certain types of blood cancers, including acute myeloid leukemia (AML) and systemic mastocytosis (SM).
Key Features:
- Blood Cancer Treatment: TAURITMO Capsule is indicated for the treatment of specific types of blood cancers, including acute myeloid leukemia (AML) and systemic mastocytosis (SM), particularly in patients with specific genetic mutations.
- Midostaurin Mechanism of Action: Midostaurin, the active ingredient in TAURITMO, is a multikinase inhibitor that targets various kinases involved in cell proliferation and survival, including FLT3 (Fms-like tyrosine kinase 3) and KIT, which are commonly mutated in AML and SM, respectively.
- FLT3-Mutated AML: TAURITMO is specifically indicated for use in patients with FLT3-mutated AML, a subtype of AML associated with poor prognosis and increased risk of relapse.
- Systemic Mastocytosis: TAURITMO may also be used for the treatment of aggressive systemic mastocytosis (ASM), mast cell leukemia (MCL), and mastocytosis with associated hematologic neoplasm (SM-AHN), conditions characterized by abnormal proliferation of mast cells.
- Fixed-Dose Capsule: TAURITMO Capsule contains a fixed dose of Midostaurin (25mg) in each capsule, simplifying dosing and ensuring consistent medication delivery.
- Oral Administration: Administered orally, usually twice daily with food, TAURITMO Capsule offers convenient dosing for patients with blood cancers, allowing them to take their medication at home.
- Combination Therapy: TAURITMO is typically used in combination with standard chemotherapy regimens for AML or as monotherapy for SM, depending on the specific disease characteristics and treatment goals.
- Clinical Efficacy: Clinical studies have demonstrated the efficacy of Midostaurin in improving overall survival and reducing the risk of relapse in patients with FLT3-mutated AML, as well as in achieving clinical responses in patients with systemic mastocytosis.
- Potential Adverse Effects: Common side effects of TAURITMO may include nausea, vomiting, diarrhea, fatigue, and rash. Patients should be monitored closely for adverse reactions and managed accordingly.
- Individualized Treatment Approach: The selection of TAURITMO as a treatment option for blood cancers should be based on factors such as disease subtype, genetic mutations, patient’s overall health status, and treatment goals.
Only logged in customers who have purchased this product may leave a review.









Reviews
There are no reviews yet.